Promotional Features
New Technology Optimizes Vitamin C: Reduced Degradation, Sustained Release and Enhanced Absorption Through Fiber Interlaced Liposomal (FIL) Ascorbic Acid Technology
Due to the demands of various food and nutraceutical industries we seek to develop products with high nutritional value by the fortification of vitamins with the aim to improve physiological function and to provide health benefits. One of the main vitamins used for this purpose is Vitamin C (Ascorbic Acid) which is potential antioxidant that helps protect our cells against the effects of free radicals.
Maintaining the stability of Vitamin C (VC) is a big challenge. VC is easily degraded in aqueous medium, at high pH, in the presence of oxygen and metal ions. Several strategies have been developed to limit the degradation processes, among them: controlling the presence of oxygen during formulation and storage, low pH, and reduction of water content using anhydrous formulations. The delivery of VC, using the oral delivery route, requires reduction of the rate of its degradation in the gut and facilitation of its absorption VC is usually administered orally in the crystalline form or as a solution, which makes it susceptible to degradation in the gastrointestinal tract. Degradation of VC can be effectively reduced by its association with the hydrophilic–hydrophobic interface, which can be provided by lipid aggregates.
So, considering factors, strategies like microencapsulation using liposomes and nanoparticles have been adopted. Liposomes are excellent delivery systems for delivering active compounds into the body. A liposomal version of biomolecules may well mimic the body’s transport system, enhancing uptake and delivery. Liposomal improves the stability and bioavailability and enhances the controlled release within the GI tract. Nevertheless, conventional liposomal formulations have many drawbacks that affect the therapeutic effect of active biomolecules and are liable to fast ‘clearance’ from the bloodstream.
Encapsulation of biomolecules like Vitamins (VC) with liposomes along with plant-based fibers will be a novel concept to enhance the stability of liposomes, which gives additional stability to the liposomes from the degradation. This will be helpful to increase the therapeutic efficiency, reduce the side effects, enhance the bioavailability of drugs.
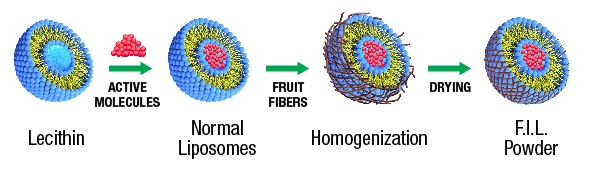
Vidya Herbs developed a unique technology called ‘Fiber Interlaced Liposomal’ (FIL) technology, which converts the liquid liposomes to a novel stabilized form of powdered liposomes. The normal liposomal products available in the market are in liquid or paste form, which may not have much stability since liposomes will break very easily in liquid formulations. The powder form is an alternate solution, but in this method the core structure of the liposome might be destroyed, due to the evaporation of water. To overcome this, soluble dietary fibers from fruits (citrus or apple) can be utilized to retain the assembly of the liposomal bilayer in its powder form. Which will help to encapsulate the bioactive molecules effectively and thus ensure sustained release.
In this technology, the dietary fibers are well integrated with the lecithin and maintain the spherical core structure even in the powder form so that the activity of liposomes will be higher. The FIL delivery vehicles reduce the VC degradation in the gastrointestinal tract, slow down its release and enhance absorption. By using the fibers, FIL products are spherical in shape, and it is less angular and much smoother. Liposomes also mitigate possible perturbances of gastrointestinal tract functioning, what enables application of elevated doses of VC for extended periods of time. All that have stimulated numerous works leading to the development of liposomal formulations of VC for varieties of applications.
Fig 1: Proposed mechanism of fiber interlaced liposomes (FIL) to form stable liposomal Vitamin C.
Hence FIL technology fulfils the requirement of equilibrium between hydrophilicity and lipophilicity of VC to improve stability and bioavailability. The equilibrium optimization would improve the bioavailability, efficacy and ensures the sustained release of the biomolecules.
Uniqueness of using fruit fibers
The fruit fiber extract used in the FIL technology has a high surface area that easily traps water and oil due to the amphiphilic nature of the fiber. Also, the fibrous composition contains lipophilic and hydrophilic ends which can bind well with lecithin to make strong reinforcement, hence can cross over the gut wall easily due to the amphiphilic nature. Because of the high surface area and composition, this fiber can be used at very low levels, which provides high functional benefits like high water holding surface area, encapsulation, emulsification, and gelling properties. FIL formulations contain high amounts of both insoluble and soluble fibers that provide functional benefits. Because of this, it improves stability, sustained release, texture, and taste first and contributes some dietary fiber second as prebiotics which will improve the immunomodulatory responses.
Sustained release study of FIL Vitamin C
The comparative in vitro release study of FIL VC and plain VC (control) was performed using the dialysis set up. Prior to the diffusion experiment, the cellulose acetate dialysis membrane (Sigma Aldrich, Molecular weight cut-off (MWCO) 12kDa) was soaked in phosphate buffered saline (PBS, pH 7.4) for 12 h. A weighed quantity of FIL VC (50 mg) was suspended in 10 mL of PBS and transferred into the dialysis bag (donor compartment). The dialysis bag was placed in a beaker containing 100 mL of PBS (receptor compartment). The setup was incubated at 120 rpm for 8 h in a 37 °C shaker incubator. A control experiment was performed simultaneously in which plain VC solution was dialyzed. A 1 mL aliquot of PBS in the receptor compartment was sampled at different time points and an equal quantity of fresh PBS was replaced in the compartment after each withdrawal. The absorbance of the samples was measured at 295 nm. The concentration of vitamin C in the samples was determined using a standard curve.
In vitro simulated digestion analysis
The FIL VC was investigated for in vitro release and digestive stability using simulated gastric fluid (SGF) and intestinal fluid (SIF) models. The liposomes were added to the respective digestive juice at 2 mg/mL and incubated at 37 °C and 120 rpm. The samples were taken at regular intervals (0, 5, 15, 30, 60, 90 and 120 min) and centrifuged at 5000 rpm for 5 min. The absorbance was measured at 295 nm and the concentration of vitamin C determined.
Results
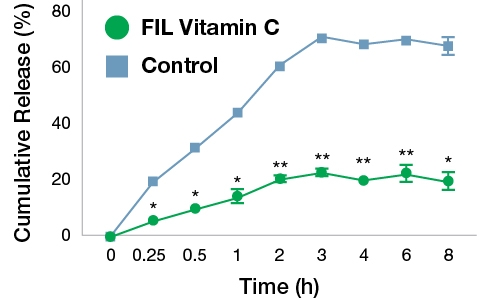
Fig. 2 shows the in vitro release profiles of VC from FIL formulation and control. There was an exponential increase in cumulative drug release up to 3 h in case of control whereas a sustained release pattern was seen in FIL formulation. The release of VC was significantly lesser in FIL formulation as compared to the control at respective time intervals (p<0.01). After 8 h, the VC released from FIL formulation and control samples were 19.31% and 67.54% respectively. These results suggest that the liposomal encapsulation inhibited the release of VC which may be favourable for prolonged release applications.
Fig. 2. Comparative release profile of VC from FIL formulation and control samples. The values are mean±SD of three independent experiments. The data were analyzed by paired t test. *p<0.01 and **p<0.001 Vs. control.
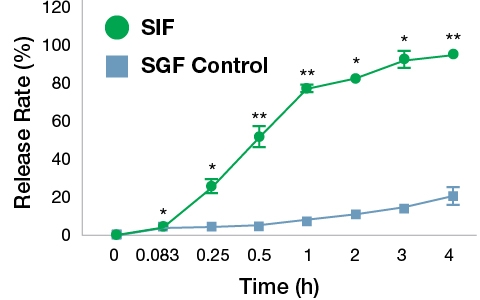
In vitro digestion of FIL VC was performed with simulated gastric and intestinal fluids. As shown in Fig. 3, the release of vitamin C from the FIL formulation in SGF was relatively slower compared to that in the SIF. The release rate of vitamin C reached 21.15% after 4 h in the SGF whereas in the SIF the rate of vitamin C release was 95.34% at 4 h. This gives the clear indication of the better stability of FIL products in the stomach and much better release and absorption in the GI tract. Usually, normal VC degrades easily in the stomach acids with the interaction of bile acids, but these results give the justification for the higher bioavailability of the FIL products.
Fig. 3. Release rate of vitamin C during simulated gastrointestinal digestion. The FIL VC sample was incubated separately in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) at 37 °C for 4 h. The release of VC was quantified at different time points. The values are mean±SD of three independent experiments. The data were analyzed by paired t test. *p<0.05 and **p<0.01 Vs. SGF.